Founded in 2007, Biophore India Pharmaceuticals Pvt. Ltd has established itself as a trusted partner for niche and complex products. With 4 US FDA and EU approved API manufacturing facilities, one dedicated intermediate facility and a world class R&D lab housing 400 scientists with varied expertise, Biophore has emerged as one of the leading API companies globally. We have consistently been in the Top 10 US DMF filers with the US FDA over the past 5 years and with most of our APIs, we are one of the fastest companies to bring them to the market enabling wider access for patients worldwide
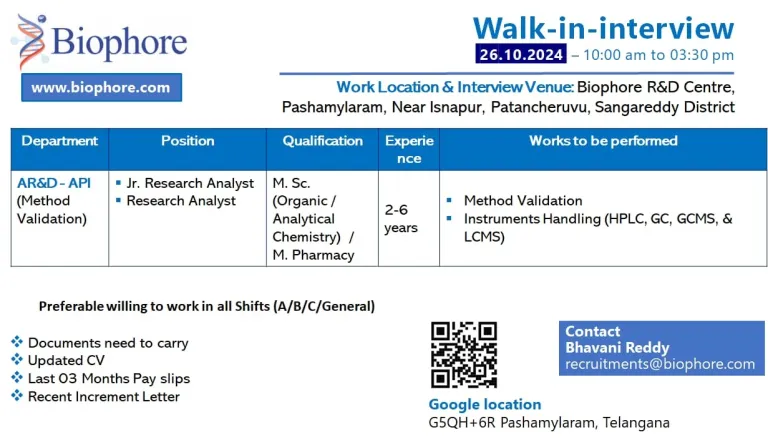
DATE AND TIME:
- Date: 26-10-2024
- Time: 10:00 AM to 03:30 PM
- Venue: Biophore India R&D Centre, Pashamylaram, Patancheruvu, Sangareddy Dist. Hyderabad.
- Share Your CV’s to : recruitments@biophore.com
job discrition:
- Formulation AR&D (Method Validation)
- Qualification: M.Sc / M.Pharm
- Experience : 02 – 06 Years
- Designation: Research Analyst / Jr. Research Analyst
Works to be performed:
- Method Validation
- Instruments Handling (HPLC, GC, GCMS, & LCMS)
Documents :
- Updated CV
- Copy of Educational certificates
- Recent Increment Letter
For More JOB Updates Join Our Whatsapp Group Click Here