Lupin Limited is one of India’s largest manufacturers of bulk actives and formulations. The principal bulk actives manufactured by it include Rifampicin, Pyrazinamide, Ethambutol (anti-TB), Cephalosporins (anti-infectives) and cardiovascular. The company also possesses competencies in phytomedicines, in which medicines are made out of plant and herbal resources supported by the discipline of modern medicine.
Date and Time:
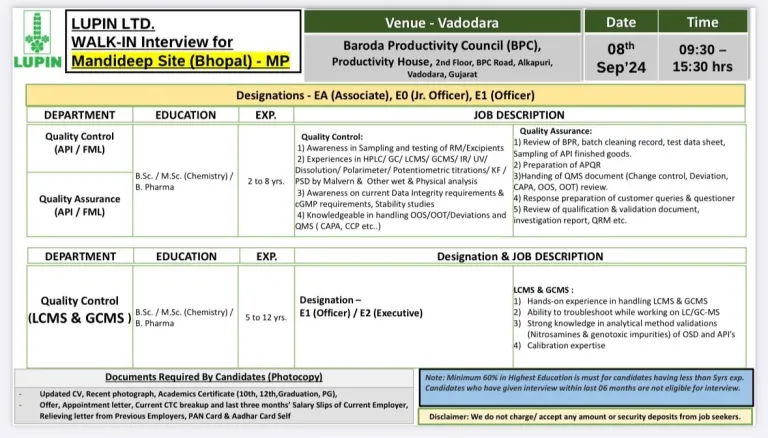
- Date: 08th Sep 2024
- Time: 09:30 – 15:30 hrs
- Venue: Baroda Productivity Council (BPC) Productivity House, Alkapuri Vadodara
Note:
- Minimum 60% in Highest Education is must for candidates having less than 5yrs exp.
- Candidates who have given interview within last 06 months are not eligible for interview.
Job Description:
- Department: Quality Control (QC) / Quality Assurance
- Division: API / FML
- Eligibility: B.Sc / M.Sc / B.Pharm
- Experience: 02 to 12 years
- Grade: Associate / Officer / Executive / Jr. Officer
Documents Required By Candidates (Photocopy):
- – Updated CV, Recent photograph, Academics Certificate (10th, 12th,Graduation, PG),
- – Offer, Appointment letter, Current CTC breakup and last three months’ Salary Slips of Current Employer Relieving letter from Previous Employers, PAN Card & Aadhar Card Self
Disclaimer: We do not charge/ accept any amount or security deposits from job seekers.
For More JOB Updates Join Our Whatsapp Group Click Here